
What is LIVTENCITY?
LIVTENCITY is a prescription medicine used to treat CMV infection and disease in adults and children 12 years of age and older weighing at least 77 pounds (35 kg) who have received a transplant, when their infection or disease does not respond to treatment with the medicines ganciclovir, valganciclovir, cidofovir or foscarnet. It is not known if LIVTENCITY is safe and effective in children under 12 years of age.

How Was LIVTENCITY Studied?
The FDA approved LIVTENCITY based on a study in adults with CMV infection after transplant (organ or HCT). CMV infections were refractory with or without confirmed resistance to one or more commonly used antiviral medicines* used in the study.
*The commonly used antiviral medicines were: ganciclovir, valganciclovir, cidofovir and foscarnet.
Patients were treated with either LIVTENCITY (400 mg, twice daily; 235 patients) or commonly used antiviral medicines (chosen by investigator; 117 patients) for 8 weeks. After completing the treatment period, patients entered a 12-week follow-up phase.
In adult patients with refractory or resistant CMV after transplant:
The main goal of the study was to evaluate how effective LIVTENCITY was at helping patients reach a low level of CMV DNA compared to commonly used antiviral medicines after 8 weeks of treatment.
Only patients 18 years of age and older were enrolled in the study. The recommended dosing of LIVTENCITY in children is based on the following:
- Clinical studies of adults taking LIVTENCITY
- Levels of LIVTENCITY in the blood are expected to be similar between adults and children 12 years of age weighing 77 pounds
- Studies showing that age and body weight had no meaningful effect on the levels of LIVTENCITY in the blood
- The course of the disease is similar between adults and pediatric patients to allow the extension of data in adults to pediatric patients
The safety and effectiveness of LIVTENCITY have not been established in children under 12 years of age.


On Treatment
LIVTENCITY was shown to be superior: More patients taking LIVTENCITY achieved a low level of CMV DNA at Week 8 compared to patients who received commonly used antiviral medicines
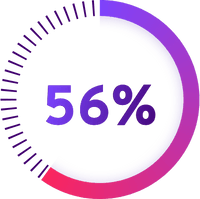
LIVTENCITY (Percent of patients with a low level of CMV DNA)
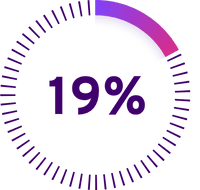
Other antiviral medicines (Percent of patients with a low level of CMV DNA)

LIVTENCITY (Percent of patients with a low level of CMV DNA)

Other antiviral medicines (Percent of patients with a low level of CMV DNA)
After Treatment
A greater percentage of patients who received LIVTENCITY maintained a low level of CMV DNA along with control of symptoms at week 16 compared to patients who received commonly used antiviral medicines

Always discuss medical questions with your transplant team.
If you need help speaking to them about CMV or LIVTENCITY, check out Tips for Talking With Your Transplant Team below.
Knowing how to take LIVTENCITY is important.